Abstract
Introduction: Forodesine, a potent purine nucleoside phosphorylase inhibitor, induces apoptosis mainly in T cells. A pivotal single-arm phase 1/2 study of forodesine at high dose was conducted in patients with relapsed Peripheral T-Cell Lymphoma (PTCL) in Japan. The results of this study at the point of data cut off (Aug 2015) demonstrated that forodesine has promising single-agent activity and led to its approval in Japan for the treatment of patients with relapsed/refractory PTCL. The overall response rate (ORR) among evaluable patients in the phase 2 part (n=41) was 22% (90% confidence interval [CI], 12-35%) (Tsukasaki, et al. ASCO 2016). Observation of all patients who continued administration at the time of data cut off was completed in February 2017. This is a report on the results using data up to the final observation of all patients.
Methods: Eligible patients, who had relapsed PTCL, confirmed by central pathology review according to the WHO classification 2008, and without major organ dysfunction, received forodesine 300 mg twice a day (BID) continuously. Tumor response was assessed with CT and PET by independent imaging review using IWC 2007 criteria. The study was conducted according to the Simon's two-stage design. Although the primary endpoint of this study was the ORR until data cut off, ORR using all data was calculated in this report. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and duration of response (DoR).
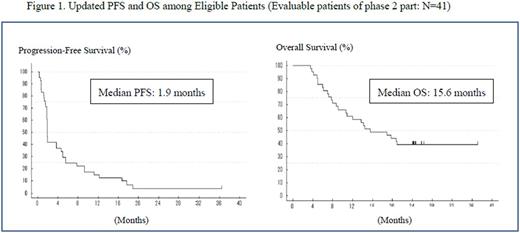
Results: The ORR in 41 evaluable patients was 24% (10 of 41; 90% CI 13.9-37.9) including 4 CR (10%) and 6 PR (15%), the lower limit of the 90% CI exceeded the 10% threshold. Median PFS and OS were 1.9 and 15.6 months, respectively (Figure 1). The 2-year overall survival was 39%. The median time to response in 10 responders was 2.8 months (range 1.8-12.8) and the median DoR was 10.4 months (95% CI, 5.9-16.0). In safety evaluable patients (N=48), serious AEs (SAEs) after dosing were reported in 22 patients (46%). As a remarkable point, 5 patients [3 angioimmunoblastic T-cell lymphoma (AITL) and 2 PTCL-NOS] with B-NHL were reported. Four out of 5 patients were positive Epstein-Barr virus- encoded small RNA (EBER+) in the tumor cells (identified using in situ hybridization), and they were diagnosed as the Epstein-Barr virus positive diffuse large B-cell lymphoma (DLBCL). In these 4 patients, 3 patients developed B-NHL during the study period, more than 200 days after the start of administration of forodesine. One patient developed B-NHL about one year after the end of administration of forodesine. Other most common SAEs (≥2 patients) were pneumonia (4 patients), pyrexia (3 patients), Pneumocystis jirovecii pneumonia (2 patients) and anemia (2 patients).
Conclusion:In the final data analysis, efficacy was confirmed against relapsed PTCL. The median DoR was 10.4 months (95% CI, 5.9-16.0 months), the responses to forodesine were durable in some patients. Compared with other PTCL options that require intravenous infusion with frequent clinic visits, the oral formulation makes forodesine easier to administer and, in turn, may be more convenient and less burdensome to patients. Forodesine would contribute as one of the reasonable options for the treatment of relapsed PTCL. Clinical Trial Information: NCT01776411.
Shibayama: Mundipharma K.K.: Honoraria, Research Funding; Novartis Pharma K.K.: Honoraria, Research Funding; Ono Pharmaceutical Co.,LTD.: Honoraria, Research Funding; Bristol-Meyer Squibb K.K.: Honoraria, Research Funding; Jansen Pharmaceutical K.K.: Honoraria; Fujimoto Pharmaceutical Co.: Honoraria, Research Funding; Takeda Pharmaceutical Co.,LTD.: Honoraria, Research Funding; Celgene K.K.: Honoraria, Research Funding. Tobinai: Janssen: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Daiichi Sankyo Co., Ltd: Consultancy, Honoraria; AbbVie: Research Funding; HUYA Bioscience: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Eisai: Honoraria, Research Funding; Servier: Research Funding; Mundipharma: Honoraria, Research Funding; Ono Pharmaceutical: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Zenyaku Kogyo: Honoraria. Tsukasaki: Takeda: Honoraria, Research Funding; Mundypharma: Research Funding; Zenyaku Kogyo: Honoraria; Celgene: Honoraria, Research Funding; HUYA: Honoraria; Kyowa-Kirin: Honoraria; DaiichiSankyo: Consultancy; Chugai/Roche: Honoraria. Uchida: Mundipharma K.K.: Research Funding; Janssen Pharmaceuticals: Honoraria. Maeda: Toko Pharmaceutical Industries: Other: Investigational drug is provided free of charge.. Nagai: Janssen, Mundipharma, Celgene, Bayer Yakuhin, AbbVie, Takeda, Chugai, Kyowa Hakko Kirin, Eisai: Research Funding; Chugai, Mundipharma, Eisai, Sanofi, Janssen: Honoraria. Hatake: AbbVie, Gilead, Celgene, Solasia, Pfizer, Bristol-Myers Squibb, Janssen, Ghugai: Research Funding; Mundipharma K.K.: Honoraria. Ando: MOCHIDA PHARMACEUTICAL: Other: Donation to institute; TOYAMA CHEMICAL: Other: Donation to institute; NOVARTIS: Other: Donation to institute; Meiji Seika Pharma: Other: Donation to institute; CHUGAI PHARMACEUTICAL: Other: Donation to institute; Sumitomo Dainippon Pharma: Other: Donation to institute; Eisai: Other: Donation to institute; Bristol-Myers Squibb: Other: Donation to institute; MSD: Other: Donation to institute; Kyowa Hakko Kirin: Other: Donation to institute; ALEXION: Other: Donation to institute; Takeda: Other: Donation to institute; Japan Blood Products Organization: Other: Donation to institute; Nippon Shinyaku: Other: Donation to institute; NIHON PHARMACEUTICAL: Other: Donation to institute; TAIHO: Other: Donation to institute; Asahi KASEI: Other: Donation to institute. Hidaka: Eisai: Honoraria; Chugai: Research Funding. Tamura: Mundipharma K.K.: Honoraria. Yamauchi: Bristol-Meyers Squibb, Chugai Pharma: Research Funding. Ueda: Mundipharma K.K.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal